Background: Multiple myeloma (MM) is associated with several cytogenetic abnormalities (CA) that influence the disease course, response to treatment and survival. Trisomies and immunoglobulin H chain translocations are primary CA while del(17p), gain(1q), del(1p) and others are secondary CA. Gain (3 copies) and amplification (>3 copies) 1q have been recognized as adverse prognostic markers and incorporated into the second revision of the International Staging System (R2-ISS). However, the role of del(1p) is less well defined, especially in the era of modern myeloma therapeutics. We aimed to analyze the outcomes of newly diagnosed MM (NDMM) patients (pts) with chromosome 1 abnormalities, mainly del 1p, treated with autologous hematopoietic cell transplant (AHCT) consolidation at The Ohio State University.
Methods: We identified and reviewed the medical records of all NDMM pts who were treated with AHCT from 1/1/2015-2/13/2019 (n=511). High-risk cytogenetics (HRC) was defined by the presence of del(17p), t(4;14), or t(14;16) similar to R-ISS; standard-risk cytogenetics (SRC) were defined as the absence of HRC. Modified HR cytogenetics (mHRC) included gain/amp 1q and/or t(14;20) in addition to HRC, while ultra high-risk (uHRC) included 2 or more mHRC risk factors.
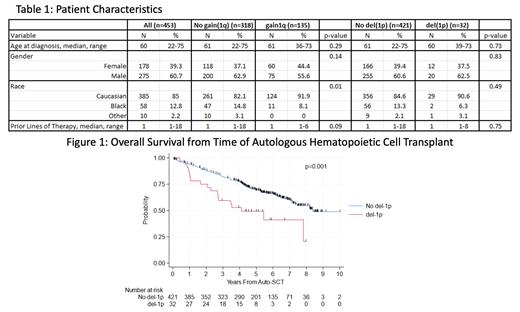
Results: The baseline pt characteristics are presented in Table 1. Of 511 pts transplanted, 453 had cytogenetic data from diagnosis. SRC were seen in 353 pts (77.9%), while 100 (22.1%) had HRC, 156 (34.4%) had mHRC, and 43 (9.5%) had uHRC. Thirty-two (7.1%) pts had del(1p) while 105 (23.2%) had gain 1q and 30 (6.6%) had amp 1q. As expected, compared to SRC pts, pts with HRC, mHRC and uHRC had higher risk of relapse or death. Patients with gain and amp 1q had inferior outcomes in terms of PFS (HR 1.35; 95% CI 1.06-1.73, p=0.016), TTNT (HR 1.84; 95% CI 1.40-2.42, p<0.001) and OS (HR 1.47; 95% CI 1.06-2.02, p=0.02), consistent with published literature. The median PFS, TTNT and OS from AHCT in pts with gain/amp 1q was 3.17 years (y), 3.95y and 7.13y, respectively, compared to 4.01y, 7.60y and 8.21y in pts without gain/amp 1q. Pts with del(1p) had inferior PFS (median 2.43y versus 3.98y; HR 1.75; 95% CI 1.16-2.64, p=0.008), TTNT (median 2.72y versus 6.17y; HR 1.96; 95% CI 1.22-3.14, p=0.005) and OS (median 4.11y versus 8.38y; HR 2.19; 95% CI 1.34-3.58, p=0.002) from AHCT compared to those without del(1p). (Figure 1).
Conclusions: In this cohort of NDMM pts that underwent AHCT, del(1p) at diagnosis was an independent predictor of shorter PFS, TTNT and OS. Despite advances in induction regimens, AHCT consolidation and maintenance therapy, del(1p) continues to portend inferior outcomes. Larger analyses are needed to validate the prognostic value of del(1p) and investigate its role in predicting outcomes in MM.
Disclosures
Khan:Janssen: Honoraria; Secura Bio: Consultancy, Research Funding; BMS: Research Funding; Amgen, Sanofi: Speakers Bureau. Cottini:The Dedham Group: Consultancy. Rosko:FDA ODAC Committee: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal